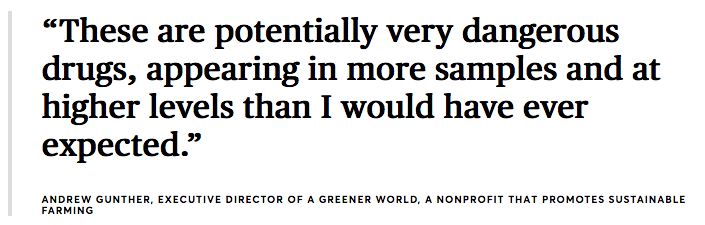Organic Consumers Association: Are Banned Drugs in Your Meat?
by Rachel Rabkin Peachman | August 29, 2018
How did they get into food? What’s known about the risks? And what can be done to keep these drugs off your plate?
Ketamine, a hallucinogenic party drug and experimental antidepressant. Phenylbutazone, an anti-inflammatory deemed too risky for human use. Chloramphenicol, a powerful antibiotic linked to potentially deadly anemia.
All these drugs are prohibited in beef, poultry, and pork consumed in the U.S. Yet government data obtained by Consumer Reports suggest that trace amounts of these and other banned or severely restricted drugs may appear in the U.S. meat supply more often than was previously known.
The data—as well as Consumer Reports’ review of other government documents and interviews with farmers, industry experts, government officials, and medical professionals—raise serious concerns about the safeguards put in place to protect the U.S. meat supply.
These concerns start with how poultry, cattle, and pigs are raised in this country. And they include questions about how the federal government tests meat from these animals, and how it investigates and enforces potential violations.
The data come from the Department of Agriculture’s Food Safety and Inspection Service, the agency tasked with ensuring the safety of the U.S. meat supply. Emilio Esteban, Ph.D., chief scientist for the FSIS, says that the results should be discounted because they came from unconfirmed screening tests.
Indeed, much remains uncertain about the test results. For one, it’s not always clear how the drugs end up in meat, though experts have ideas, including contaminated feed and intentional misuse. There are also questions about whether the amounts of drug residue found in the samples pose risks to humans, in part because little research has been done to investigate that possibility.
Still, CR’s food safety scientists, and other experts we consulted, say the results are meaningful and concerning.
“These results are credible enough that you would expect the government to take the warning signs seriously,” says James E. Rogers, Ph.D., who was a microbiologist at the FSIS for 13 years before becoming director of food safety research and testing at Consumer Reports. “You would hope the results would prompt the agency to look into why these drugs may be present, what risks they could pose, and what could be done to protect consumers.”
A Debate Over Data
The data CR evaluated were obtained through a Freedom of Information Act request as part of an ongoing lawsuit brought by several food safety organizations against Sanderson Farms, one of the nation’s largest chicken producers. The groups allege that Sanderson makes misleading claims about its chicken being natural and free of antibiotics. The company denies that its labels are misleading.
But the data raise questions about more than just one company or class of drugs.
Hundreds of samples of poultry, beef, and pork appeared to show residue of drugs that the government says should never be used in food animals. Other samples had evidence of drugs that must be out of an animal’s system by the time it is slaughtered. The samples came from producers large and small, and included meat destined for supermarkets, restaurants, hospitals, schools, and elsewhere.
Yet FSIS officials have taken little if any action based on the data. When asked to explain why not, Esteban, at the FSIS, said the samples didn’t meet several criteria used by the agency to decide when a sample requires follow-up testing.
For example, he said that some results came from tests that have never been validated for certain animals or drugs. And, he said, in many cases the results were below a level that the agency considers worrisome. The agency subsequently released a second set of data that, it says, reflected test results after those criteria had been applied, and that made the initial results invalid. In a written response, an agency spokesperson said, “Reporting preliminary unconfirmed data will be misleading as these data do not represent any public health risk to consumers.”
Consumer Reports’ food safety scientists disagree.
They point out that the testing and methodology used by the FSIS to check for drug residue in meat is rigorous, capable of detecting even very small amounts of the drugs. In fact, they say that the test the agency uses to confirm results is the same one it uses to screen for drugs in the first place.
CR’s scientists also note that the FSIS cutoffs seem much higher than those used by other scientists, even in other government agencies. And in documents provided to CR, the FSIS acknowledged that even for drugs that should not be in meat, the agency sets its cutoffs above what the test can measure.
Finally, CR’s analysis of the data identified many samples with results above even the FSIS’ own cutoff.
The Science of Detection
The results CR analyzed, covering tests performed from October 2015 through September 2016, come from nearly 6,000 samples selected at random by the FSIS at slaughterhouses around the country. The samples were then sent to a lab and tested with a device capable of measuring dozens of compounds at once, often down to the parts-per-trillion level.
That device, which the FSIS began using in 2012, replaced technology that could measure fewer substances and only to the parts-per-million level, says Parthapratim Basu, D.V.M., who worked at the FSIS for 35 years before retiring as chief public health veterinarian in January 2018. (He is currently consulting for a law firm representing Sanderson Farms.)
Basu and other experts say the FSIS set its higher cutoffs—what the agency calls its minimum level of applicability (MLA)—partly in response to this new equipment.
“Analytical equipment has gotten so sensitive that it’s possible to detect things that you wouldn’t have 20 years ago,” says Robert Poppenga, Ph.D., a professor of veterinary toxicology at the California Animal Health & Food Safety Lab at the University of California, Davis, who has worked with the FSIS. Using the MLA, he says, gives “authorities some flexibility, and if they do find something at a very, very low level, they don’t necessarily have to take regulatory action.”
“Is it a perfect system?” Poppenga says. “Probably not. But we don’t have the resources to do a risk assessment for every possible chemical that could possibly be there.”
Some experts, however, worry that by relying on higher cutoffs, the FSIS may overlook possible health threats. Some research, including a 2015 review in the Journal of Veterinary Science & Toxicology, suggests that long-term exposure to low levels of drug residue in meat could increase the risk of cancer, fetal harm, antibiotic resistance, and more.
Experts also worry that the FSIS could be failing to fully investigate the problem. “Any responsible agency should want to understand how widespread contamination is,” says Charles Benbrook, Ph.D., a visiting scholar in the Bloomberg School of Public Health at Johns Hopkins University, who consulted with the food safety groups in the Sanderson lawsuit. “Certainly it should have resulted in aggressive action . . . to figure out why these drugs are getting into animal products.”
Inconsistent Regulation
Other U.S. government agencies seem to be concerned about these drugs even at levels below the cutoff used by the FSIS.
The Food and Drug Administration, for example, has blocked shrimp from Malaysia because it contained chloramphenicol at levels as low as 0.3 parts per billion. By contrast, the FSIS regulatory cutoff for chloramphenicol in meat is 3 ppb, an amount 10 times as high.
The Environmental Protection Agency has also taken issue with the FSIS cutoffs, according to a 2014 report from the Government Accountability Office. It noted: “EPA expressed its concerns to FSIS that the relatively high FSIS minimum levels of applicability hampered EPA’s ability to accurately estimate exposure” to pesticides in food.
Other countries seem to be concerned about these drugs at levels below the FSIS threshold as well. After a scandal involving beef contaminated with horse meat rocked Europe in 2013, regulators there worried that consumers could be exposed to phenylbutazone, a drug approved for use in horses. In response, regulators reaffirmed that meat with phenylbutazone was unfit for human consumption, publishing cutoffs ranging from 1 to 11 ppb. By contrast, the FSIS sets its cutoff for the drug in pork at 50 ppb.
Why Standards Matter
CR’s scientists and several food safety experts we consulted—including Basu, the former FSIS official—say that instead of its less stringent cutoff, the FSIS should use more widely accepted scientific standards.
That includes the limit of quantitation (LOQ), which is the lowest amount of a substance that an instrument and testing procedure can reliably measure. “I find that very disturbing that [the FSIS has] different standards,” says Ronald Baynes, Ph.D., director of the Center for Chemical Toxicology Research and Pharmacokinetics at North Carolina State University’s College of Veterinary Medicine.
But the FSIS says it does not use LOQs. So CR’s scientists, using other government documents and interviews with experts, made their best estimate of appropriate, conservative cutoffs for some of the most concerning drugs. With that information, they identified samples in which drug residues appeared to be truly present, and not mere statistical or instrument noise.
The results of that analysis (see the chart below), which focused on four particularly worrisome drugs, are troubling, say CR’s scientists and some outside experts.
“I’m floored by these results,” says Andrew Gunther, a food animal production expert and executive director of A Greener World, a nonprofit that promotes sustainable farming. “These are potentially very dangerous drugs, appearing in more samples and at higher levels than I would have ever expected.”
Industry groups, however, stand by the FSIS. Conclusions based on preliminary results “would amount to fearmongering and needless alarm,” says Ashley Peterson, Ph.D., senior vice president of scientific and regulatory affairs at the National Chicken Council, echoing statements from other groups representing beef, pork, and turkey producers.
How Do Banned Drugs Get Into Meat?
That’s not always clear, but experts offer some possibilities.
Background exposure. With drugs prescribed so widely in humans and livestock, trace amounts from runoff or excrement can end up in soil and water, says Gail Hansen, D.V.M., a veterinarian who focuses on public health. That residue could reach the water or feed that animals consume, and ultimately be detected in meat.
Another explanation is that certain drugs may occur naturally in the environment. Chloramphenicol, in fact, was originally developed from a compound found in soil.
Improper use. Drug residue can also be found in meat if an animal was given the wrong dose or not enough time passed before slaughter to let the drug clear the animal’s system.
Counterfeit drugs. A 2017 report, “Illegal Veterinary Medicines,” by the nonprofit group Health for Animals noted that counterfeit veterinary drugs, which mainly come from China and India, could threaten human health “through consumption of food from animals treated with these products.” And last year the FDA warned about U.S. ports receiving shipments of several drugs—including chloramphenicol, ketamine, and phenylbutazone—that were labeled for manufacturing but could be intended for unapproved veterinary uses.
Contaminated feed. Several industry insiders say this is a particularly likely explanation. Jonathan Buttram, a farmer who raised chickens for many years, says that feed often contains parts of other animals, such as cattle. And animals turned into feed could be more likely to have been sick and treated with drugs prior to slaughter, says Jennifer Burton, D.V.M., a veterinarian who focuses on sustainable farming. Residue from these medications could make it into feed, and then into the animals that consume it.
Contamination could also occur if a drug approved for nonfood animals gets introduced into feed for a food animal, perhaps because a feed mill is not cleaned well between uses or because feed bags get mixed up, Hansen says.
Intentional misuse. Farmers we contacted could not or would not point to specific instances, but some said that producers sometimes misuse veterinary drugs to speed growth, increase lean protein, or treat sick animals.
“If you’re asking, ‘Do people do it?’ I would say, ‘Do people speed?’ ” says Will Harris, owner of White Oak Pastures, an organic farm in Bluffton, Ga. “The answer is yes—if they think they can get away with it.” He points out that most meat never gets checked, so producers sometimes “succumb to temptation, especially when there is a financial reward.”
For example, cattle that can’t stand on their own are not allowed to be used for meat. So, Basu says, lame cattle are sometimes given phenylbutazone—a painkiller—shortly before slaughter, so they can “get the animal through the slaughterhouse gates without anybody looking closer.”
There also appears to be an active black market for veterinary drugs. In March 2017, a Virginia man who pleaded guilty to selling veterinary medications illegally said during his sentencing hearing that he was hardly alone: “It didn’t make what I done right, but this stuff has been traded out of the back of vehicles forever.”
The Struggle for Enforcement
FSIS testing does sometimes lead to companies being cited for violations. But these citations are usually for drugs, mostly antibiotics, that are approved for use in animals and simply exceed their residue limits.
Very few violations are for drugs that should never be in meat. Yet CR’s analysis, which focused on just four drugs, identified numerous samples that appeared to contain detectable amounts of these zero-tolerance drugs, both above and below the FSIS regulatory threshold.
Charles Benbrook, the Johns Hopkins researcher, suspects that the FSIS may set high thresholds in part because it doesn’t have the resources to deal with the extra violations that could result if it used lower levels.
Other experts point to what they see as additional shortcomings in FSIS testing and enforcement practices.
For example, Baynes questions why the FSIS has not validated its test for all the drugs CR looked at, especially phenylbutazone in beef, because it’s known that the drug has been misused in cattle, and other scientists have been able to validate similar tests in beef.
And Basu and Baynes worry that the FSIS tests often focus on the wrong parts of animals. To detect improper drug use, they say, you should sample kidneys or livers, where drugs tend to accumulate. Yet the FSIS more often tests muscle. The FSIS says it tests muscle because that’s what consumers usually eat. But Basu and Baynes say that also means that improper drug use is less likely to be discovered.
Even when violations are reported, Basu believes the FDA’s penalties are often ineffective. FDA officials say that penalties can include warning letters, injunctions, seizures, and placing repeat violators on a publicly reported list.
But that rarely leads to changes in how meat producers operate, Basu says. “I’ve been to farms where they are proud to get letters from FDA,” he says. “They cover the holes in the barn with the letters. And just keep on doing it.”
What Consumers Can Do
CR’s food safety experts don’t think that the concerns raised in this investigation mean you should give up or necessarily cut back on meat. The findings are too uncertain and the potential risks still unknown.
But research suggests that many Americans eat more meat than recommended for good health and that reducing meat consumption can be better for the environment. The potential problems identified here may be enough for some to consider eating less meat.
The data CR analyzed are not robust enough to say whether particular companies are more likely than others to have drug residue in meat.
Nor was there enough information to say for certain that organic meat is less likely to have drug residue. Still, organic farms are subject to additional monitoring from the government and independent organizations, and federal law generally requires them to raise their animals without drugs or other chemicals. “The USDA Organic seal can’t guarantee that the meat will be drug-free, but the additional rules and oversight do increase the odds,” says Charlotte Vallaeys, Consumer Reports’ organic food expert.
Finally, if you work in meat or feed production, and know of the misuse of veterinary drugs, we want to hear from you.
Additional reporting by Lea Ceasrine.
Banned Drugs: What the Data Shows
CR’s analysis of data from the Food Safety and Inspection Service, a branch of the Department of Agriculture, suggests that banned or restricted drugs may appear in the U.S. meat supply more often than was previously known. Below are descriptions of four of these drugs, along with estimates of how many meat samples tested by the FSIS were above a cutoff used by the agency to determine when a drug is present in the meat, as well as how many were above a cutoff that CR’s scientists and outside experts say is more scientifically justified.
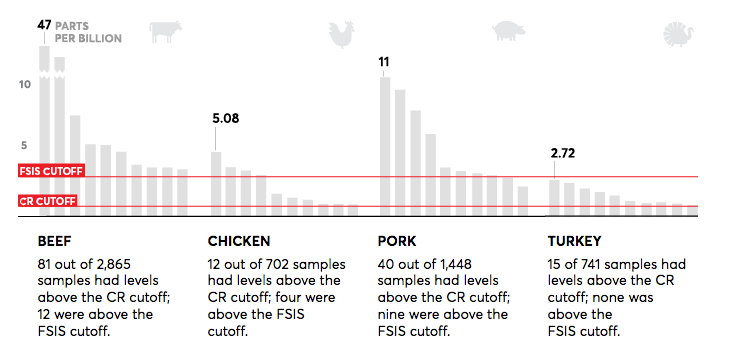
Chloramphenicol in Meat Samples
This antibiotic, at any exposure level, can trigger life-threatening aplastic anemia, or the inability to produce enough new blood cells, in 1 in 10,000 people. For poultry, beef, and pork samples combined, 148 (2.6 percent) of 5,756 samples had chloramphenicol levels above CR experts’ cutoffs; 25 were above the FSIS cutoff. Below are the 10 highest samples for beef, chicken, pork, and turkey.